Solar fuel-splitter stepped-up with new material
 Researchers have developed a very promising prototype of a new solar cell, which produces clean hydrogen gas fuel from liquid water.
Researchers have developed a very promising prototype of a new solar cell, which produces clean hydrogen gas fuel from liquid water.
The electricity produced by a solar cell can be used to set off chemical reactions. The ability can be harnessed to create new solar fuels - a hugely promising replacement for pollutant-laden fossil fuels.
One method is to split liquid water using solar electricity (through electrolysis), to produce hydrogen gas that can be used as a clean fuel in the chemical industry or combusted in fuel cells.
Researchers around the world are targeting their studies at a semiconductor material that is able to both convert sunlight into an electrical charge and split the water, all in one; a kind of 'solar fuel cell'.
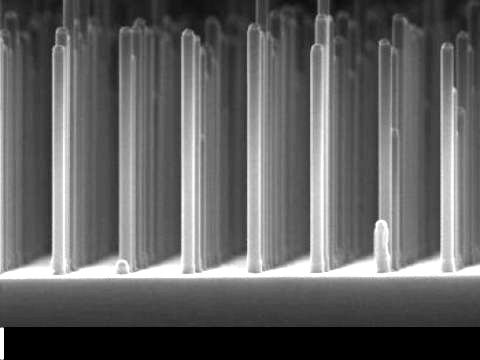
Excitingly, Dutch engineers have now used the material gallium phosphide in the form of very small nanowires to help boost their yield by a factor of ten, compared to previous designs, while using ten thousand times less precious material.
The team from the Eindhoven University of Technology (TU/e) and FOM Foundation see gallium phosphide (GaP), a compound of gallium and phosphide, could be their dream material.
GaP has good electrical properties but the drawback that it cannot easily absorb light when it is a large flat surface as used in GaP solar cells.
The researchers have overcome this problem by making a grid of very small GaP nanowires, measuring five hundred nanometers (a millionth of a millimetre) long and ninety nanometres thick.
This immediately boosted the yield of hydrogen by a factor of ten to 2.9 percent. This was a record for GaP cells, despite being some way short of the fifteen per cent achieved by silicon cells coupled to a battery.
According to research engineer Eric Bakkers, it is not just about the yield.
While there is still a lot of scope for improvement, he points out that: “For the nanowires we needed ten thousand less precious GaP material than in cells with a flat surface”.
“That makes these kinds of cells potentially a great deal cheaper,” Bakkers says.
“In addition, GaP is also able to extract oxygen from the water - so you then actually have a fuel cell in which you can temporarily store your solar energy.
“In short, for a solar fuels future we cannot ignore gallium phosphide any longer.”
More details are available in the report - Efficient water reduction with gallium phosphide nanowires.








 Print
Print