Animal cells get plant skill
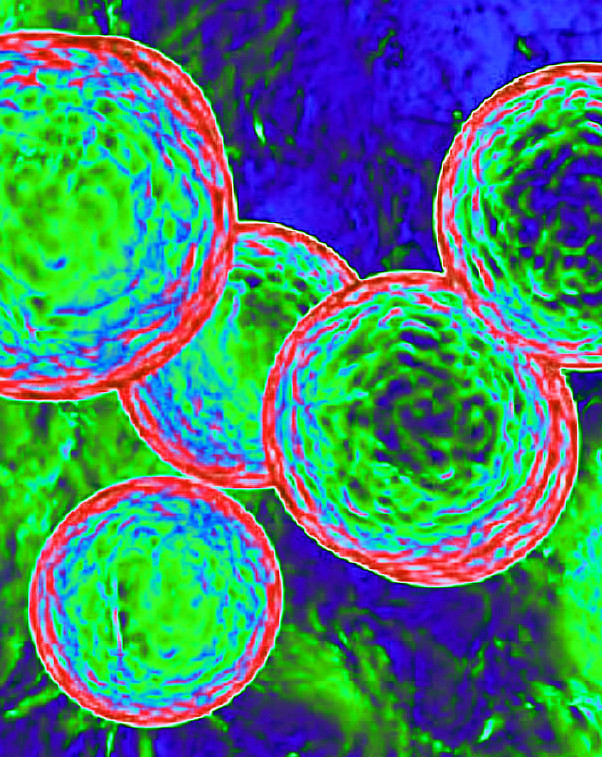 Scientists have modified animal cells to photosynthesise like plants.
Scientists have modified animal cells to photosynthesise like plants.
At the University of Tokyo, researchers have developed hybrid animal cells that gain energy from sunlight - a trait normally exclusive to plants.
By introducing chloroplasts, the energy-producing organelles in plant cells, into animal cells, the team has opened new possibilities for bioengineered tissues, lab-grown meat, and sustainable energy solutions.
In nature, plants and algae use chloroplasts to convert sunlight into energy, while animal cells rely on mitochondria to process chemical energy from food.
The new study, however, introduces chloroplasts from red algae into hamster cells, creating animal cells that maintained photosynthesis for up to two days, defying the team’s expectations that the chloroplasts would quickly deteriorate.
“We thought that the chloroplasts would be digested by the animal cells within hours…what we found was that they continued to function for up to two days,” said Professor Sachihiro Matsunaga, the study’s corresponding author.
The researchers confirmed the presence and function of the chloroplasts by observing chlorophyll, a compound essential for photosynthesis that fluoresces under certain lighting.
This fluorescence allowed the team to detect photosynthetic electron transport, the process of converting light to chemical energy within the hybrid cells.
This discovery holds significant implications for tissue engineering, particularly for lab-grown organs and meat production.
Presently, lab-grown tissues face challenges due to oxygen shortages that restrict growth.
Matsunaga explained that “mixing in chloroplast-implanted cells could enable oxygen to be supplied to the cells through photosynthesis, by light irradiation, thereby improving conditions inside the tissue to enable growth”.
In a first for animal cell research, the chloroplast-enhanced cells showed increased growth rates, suggesting they may have provided a valuable carbon source.
This could further support the scalability and viability of bioengineered tissues, potentially revolutionising the production of lab-grown meats and other artificial organs.
Previous attempts had succeeded in transplanting chloroplasts into yeast cells, but this achievement represents a major leap for animal cell engineering.
The hybrid cells, dubbed “planimal” cells, may even support sustainable energy solutions.
“We expect planimal cells to be game-changing cells, which in the future can help us achieve a ‘green transformation’ to a more carbon-neutral society,” Matsunaga said.
Published in the Proceedings of the Japan Academy, the study could be a major advance in biotechnology.
Researchers plan further studies to investigate how these hybrid cells can continue to support oxygen production and sustainable energy generation in lab-grown tissues and organs.








 Print
Print